Two-Step Cyanomethylation Protocol: Convenient Access to Functionalized Aryl- and Heteroarylacetonitriles
Accesing (hetero)arylacetonitriles using no cyanide at all.
If you search through this blog you will find other entries dealing with new methods for the preparation of phenylacetic acids. These building blocks are quite used in medicinal chemistry, and it seems that you never have enough variety in the shelf. Today you need with a fluorine, and tomorrow also with a chlorine. Typical precursors of these acids are the phenylacetonitriles, which can be used to prepare many different things including amides, amines, ketones and heterocycles.
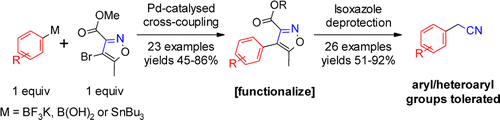
The chemists in Eli Lilly have the same needs or they have too much spare time, because Lindsay-Scott (Eli Lilly UK) has published a paper dealing with the preparation of aryl- and heteroarylacetonitriles using an anti-atom economy building block. But the idea works nicely. In short, they took a 2-bromoisoxazole and coupled it to suitable boron partners (trifluoroborates and boronic acids). The bromoisoxazole has an ester moiety that improves the stability of the molecule and allows to trigger the decomposition in basic medium to yield the desired products.
The protocol tolerates the presence of fluorine, chlorine (more or less), nitriles, amines, ethers, thioethers and others in the aryl ring, with yields from moderate to good, using coupling conditions developed by Molander. However, it does not work with heteroaryltrifluoroborates. So they turned to the corresponding stannanes using conditions inspired by Fu. Again, success with good yields. The examples include pyridines, thiazole, benzothiophene and indole. The deprotection of the isoxazole is carried out using 3 equivalents of KF in DMF/H2O.
No idea about you, but we have a project right now where this protocol comes in handy.
Org. Lett., Article ASAP. See: 10.1021/ol503479g