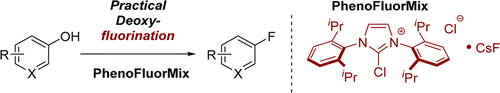
PhenoFluorMix: Practical Chemoselective Deoxyfluorination of Phenols
Improved reagent for the direct conversion of phenols into fluorides.
In a previous blog entry (see OH? F!) we commented a paper by Ritter dealing with a new fluorinating agent, PhenoFluor (N,N’-1,3-bis(2,6-diisopropylphenyl)-2,2-difluoroimidazolidene), capable to transform phenols into aromatic fluorides. This transformation is quite interesting for med chemists. In our own labs, is not uncommon having a series where you have to prepare the analogs with OMe, OH and F in the same position. So if you can prepare the OMe in quantity enough, we use that same product to prepare the phenol by demethylation. And from that point, the fluoride by deoxyfluorination.
Ritter (Harvard University, MA, USA) has published a new paper addressing some problems of PhenoFluor and presenting a new, more convenient reagent that effects the same transformation: PhenoFluorMix. To summarize, the new reagent is not moisture sensitive and is available on larger scale (and I hope it is also cheaper). In the 20 examples presented in the paper, the yields are usually good. Half of the examples involve heterocycles (pyridines, pyrimidines, isoquinolines and so) with very acceptable yields. The reagent is available (or will be, because the site displays a message of ‘no current info available’) through Sigma-Aldrich.
Org. Lett., Article ASAP. See: 10.1021/ol5035518