Flow Chemistry
New year, new tricks. In a previous issue of our newsletter we have talked about one of the latest developments in synthetic organic chemistry, flow chemistry and continuous reactors. GalChimia is entering now the wonderful world of Flow Chemistry and we want to share with you our first succesful experiences using this technique.
Though in fact continuous reactors have been known and used for a long time (for example in the petrochemical industry), only very recently they have made their way into traditional organic synthesis. As a company devoted to provide services in organic synthesis to the pharma, chemical and biotech industry, we are simply fascinated by a technology with such interesting possibilities. In many projects we have faced problems derived from the scale up: impurities formed on extended reaction times; low yields due to stirring issues; exothermic reactions which become run away reactions when going from milligrams to a few grams… Sounds familiar?
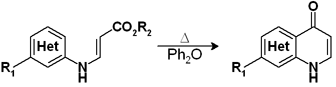
So let’s say that the first time we tried a flow chemistry equipment we had excellent candidates for the trial. The first one was a thermal cyclization of a 3-(heteroarylamino)acrylate. This reaction had to be performed in Ph2O in order to get the activation temperature. The Ph2O is no the easiest solvent to get rid of, and to top it all the reaction crude was a dark syrup which had to be thoroughly purified to obtain the product in a 30% yield.
For the screening of new conditions we were interested in a) changing the solvent and b) doing the reaction as fast as possible to avoid the syrup effect. We tried a couple of solvents and discovered that the cyclization needed temperatures between 210-240 °C. Residence time and temperature in the heating coil were adjusted to provide time enough for the product at such temperatures to cyclize and leave before decomposing. The product obtained was so pure that in fact we had a problem when it crystallized at the lower temperatures in its way to the collector. A simple trick avoided this, so we ended with an excellent procedure for preparing the product in big quantities without the need to purify it.
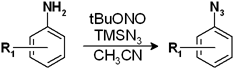
Our second target was an aryl azide used as starting material for the preparation of a tetrazole. While the preparation of the tetrazole itself was uneventful, the scale up of the azide was really troublesome. Though the temperature of the reaction was strictly controlled, at least twice the reaction had projected outside the vessel. Well, our work is not making volcanoes, but molecules, so fixing the reaction was imperative.
We followed similar principles to those used in the previous reaction. What was the critical temperature? There were some solubility problems of the azide in the solvents we wanted to try? After some experimentation we found that running the reaction in the same solvent, acetonitrile, at 80 °C for 5 minutes or 90 °C for 4 minutes generated a perfect reaction, from TLC spot to TLC spot. The nitrogen being generated and the heat of the exotherm were being taken care of nicely by the flow system, so no more projections! We finally tried the reaction with 2 g of substrate and we are happy to say that the reactor did not made it to the orbit. The 2 g were consumed in 30 minutes, so we could run the reaction with 100 g in 25 h without safety issues.
As conclusion, we improved two troublesome reactions to avoid impurities generated by extended reaction times and fixed security issues. Not bad at all for a day’s work.