Gold-Catalyzed One-Pot Cascade Construction of Highly Functionalized Pyrrolo[1,2-a]quinolin-1(2H)-ones
Synthesis of a fused heterocycle using a gold catalyst.
Gold chemistry is one of those expanding fields in organometallic chemistry. Leaving aside the exotic factor (hey, I am doing reactions with gold!), this metal provides some unique and interesting transformations.
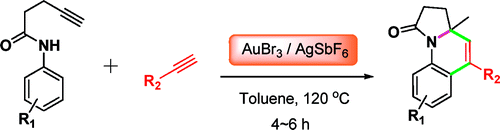
This paper by Liu (Shangai, China) is a good example. They applied a gold-based methodology to the synthesis of a fused heterocyclic system, in this case pyrrolo[1,2-a]quinolines. The method requires the use of two alkynes, a 4-pentynamide, and an aryl alkyne, which in a one-pot process are linked to form the final compound. In a typical reaction, a tolylpent-4-ynamide and an aryl alkyne react with AuBr3 and AgSbF6 in toluene at 120 °C for 4 h, giving the products in yields from good to very good, not bad for a reaction where two C–C bonds and one C–N bond are formed in just one operation. Although all 12 examples provided are based on common aryl products, I wonder whether some heterocycles could be introduced…
J.Org.Chem., ASAP. See: 10.1021/jo901418m