Pd(II)-Catalyzed Olefination of Electron-Deficient Arenes Using 2,6-Dialkylpyridine Ligands
An alternative method for meta C–H activation.
The introduction of any group in meta position is usually troublesome and tedious. In the very first issue of our newsletter, we presented a paper by Hartwig on the meta halogenation of 1,3-disubstituted arenes using an iridium catalyst (see issue 01). And things are changing.
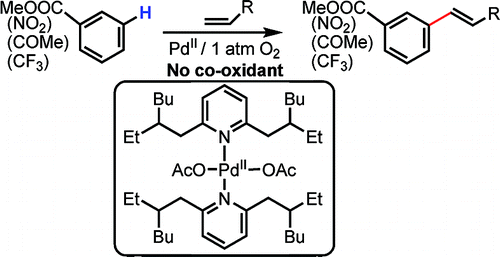
This paper by Yu et al. (Scripps, CA, USA) presents a Heck-like olefination over an arene, not an aryl halide, in the meta position. Under typical reaction conditions, the alkene, Pd(OAc)2 (10 mol%), a ligand (20 mol%), and Ac2O under 1 atm of O2 in 2 mL of the selected arene at 90 °C gives a reaction mixture where the meta/para compounds are present in a 3:1 relation. The reaction can be done also with common solvents instead of neat, a good point as many arenes are solids.
The method still needs some refinements. A richer reaction mixture would be desirable, electron-rich arenes are also interesting substrates and the ligand is not commercially available, but the authors show the potential of the new methodology by preparing an advanced substrate, a 1,2,4,5-substituted arene, from a 4-substituted benzoate.
J. Am. Chem. Soc., 2009, 131 (14), pp 5072–5074. See: 10.1021/ja900327e