Triethanolamine-Mediated Palladium-Catalyzed Regioselective C-2 Direct Arylation of Free NH-Pyrroles
Arylation of pyrroles using a phosphine-free protocol.
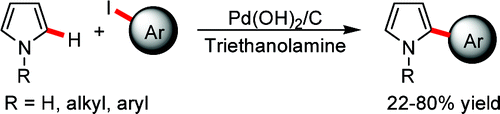
‘C-H activation is one of the hottest topics in Organic Synthesis’. If this sounds familiar to you it is because we have said it in another article of this Top Ten. This paper by Jafarpour (University of Tehran, Iran) is a very interesting work related with the direct arylation of pyrroles. Probably the most known substrate with a pyrrole scaffold is Atorvastatin, the blockbuster drug, and many other pyrroles are biologically active, so a route for the functionalization of pyrroles in the C-2 is quite interesting, specially if it can be applied on industrial scale.
The method developed by Jafarpour emphasizes some green chemistry points: recoverable solvent, cheap catalyst, etc. In a typical protocol, the pyrrole and an aryl iodide (3.0 equiv) are mixed with Pd(OH)2/C (10 mol%) and solved in triethanolamine. The mixture is heated at 100 °C for 24 h. The triethanolamine acts as solvent and base, and has been recently demonstrated that can be used as an efficient and reusable medium for other palladium-catalyzed reactions, the Heck coupling. The method has some drawbacks: First, aryl iodides must be used, so atom economy is low and substrates are more expensive. Second, yields obtained range from good to very good, but always under 80%. The reaction needs 24 h, but seeing the solvent and conditions I wonder if microwave heating cannot enhance the reaction rate. One strong point of the method is its ability to couple sterically hindered iodoarenes.
J. Org. Chem., 2010, 75 (9), pp 3109–3112. See: 10.1021/jo902739n