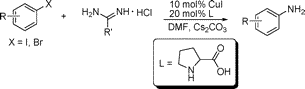
Copper-Catalyzed Synthesis of Primary Arylamines via Cascade Reactions of Aryl Halides with Amidine Hydrochlorides
Sometimes, the fastest way is the shortest way. Now, also true for arylamines.
There are some widespread reactions that you always wish they would go a bit further. For example, aminative reduction usually fails when you try to convert a carbonyl compound into a primary amine, because ammonium acetate works only in a few cases. The Buchwald–Hartwig reaction, the metal-catalyzed partner of the aminative reduction, has similar shortcomings. If you need a primary amine, you can try benzylamine or similar, but you need to a deprotection step.
The groups of Fu and Qiao (Tsinghua & Beijing, China) have found a way to overcome this limitation and obtain directly primary amines through the Buchwald–Hartwig protocol. Their trick involves using a new ammonia surrogate and a new ligand. Well, in fact both the surrogate and the ligand are old, but this use is new. As an example, 3-nitroiodobenzene reacts with acetamidine hydrochloride using CuI (10 mol%), L-proline (20 mol%) with Cs2CO3 in DMF at 110 °C for 10 h to yield the corresponding aniline in a 92%. No hydrolisis step is required. The protocol can be applied to aryl bromides and iodides, with better results for iodides, with yields ranging from 64% to 94%.
J. Org. Chem., 2008, 73 (17), pp. 6864–6866. See: 10.1021/jo800818e