Direct Arylation of Azine N-Oxides with Aryl Triflates
Another demonstration of C–H activation using N-oxides of azines and azoles.
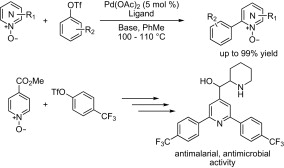
In the previous issue, we included a paper on the palladium-catalyzed C–H activation of N-oxides of azines and azoles. The paper we present here is a follow-up by the same author, Fagnou (Ottawa, Canada) expanding previous work so that aryl triflates can be used and differently diarylated products can be obtained. Two reaction protocols are described: conditions A, using Pd(OAc)2 (5 mol%), HP(Cy3)BF4 (10 mol%), Rb2CO3 and PivOH (40 mol%) in toluene at 100 °C; and conditions B, using Pd(OAc)2 (5 mol%), HP(tBu2Me)BF3 (10 mol%), K2CO3 and PivOH (30 mol%) in toluene at 110 °C. Both conditions are compared, and conditions B are shown to be better than conditions A. This fact is used to prepare asymetrically disubstituted azines in excellent yield.
Tetrahedron, 2009, 65, pp 4977–4983. See: 10.1016/j.tet.2009.03.077