An Efficient and General Method for the Heck and Buchwald–Hartwig Coupling Reactions of Aryl Chlorides
Efficient Heck coupling and Buchwald-Hartwig amination of aryl chlorides.
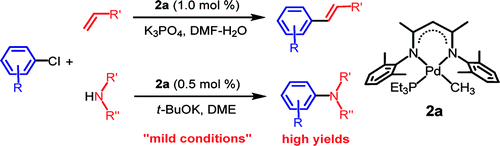
Honestly, I do not feel exactly comfortable including this paper, because someway I feel the authors are cheating. This paper by Jin et al. (Inha University, South Korea) shows the results for the Heck and Buchwald-Hartwig coupling of non activated aryl and heteroaryl chlorides. The trick is using a special catalyst, a b-diketiminatophosphane palladium complex. This complex is not commercial, but is synthetized easily in three steps from 2,4-diketopentane. The authors report 32 examples of the Heck coupling and 9 examples of the Buchwald-Hartwig amination, all with excellent results on all type of substrates, including heterocycles. What I miss are examples of cloroarenes with another halide, that is, bromochloroarenes and chloroiodoarenes, so we can see selectivity of the reaction (it is not commented on the paper) and assess if you can have an orthogonally activated arene for couplings using two different catalytic systems. Hmmm….
Org. Lett., Article ASAP. See: 10.1021/ol202177k
Freak Corner. Inha University is located in Incheon city, the site of the Battle of Inchon during the Korean War. This battle began on September 15, 1950, with an amphibious invasion involving some 75,000 troops and 261 naval vessels. The battle ended on September 19, and led to the recapture of the South Korean capital Seoul two weeks later. The landing at Incheon was not the first large-scale amphibious operation since World War II. That distinction belongs to the July 18, 1950, landing at Pohang, but it is the biggest landing since D-Day.