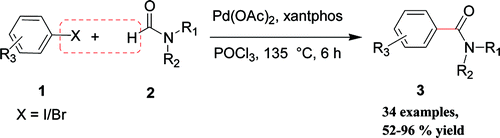Palladium-Catalyzed Carbon-Monoxide-Free Aminocarbonylation of Aryl Halides Using N-Substituted Formamides as an Amide Source
An aminocarbonylation protocol to introduce directly amides.
This paper by Bhanage et al. (Institute of Chemical Technology, Mumbai, India) is strange. I mean, this is the first time I have seen a Palladium coupling performed in a medium with POCl3. The protocol goes for a formal carboxylation, which generates amides as final products. The trick is performed by the generation of a Vilsmeier-type intermediate from the starting amide, which in turns is coupled by the palladium to generate the desired compound. You can use almost any amide, from the simple DMF to more interesting things from the medicinal chemistry point of view, so you can save steps by preparing directly the amides instead generating the acid and then going with one of the amide formation protocols. The reaction can be performed with bromo- and iodobenzenes as starting substrates. About 20 examples are described, with excellent yields.
J. Org. Chem., 2011, 76 (13), pp 5489–5494. See: 10.1021/jo200754v