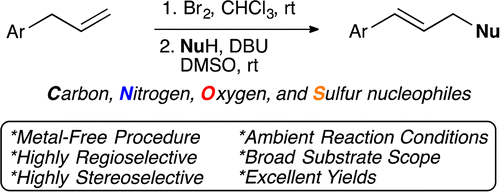
Metal-Free, Regio- and Stereoselective Synthesis of Linear (E)?Allylic Compounds Using C, N, O, and S Nucleophiles
Using terminal alkenes to prepare allylic acetates and related beasts.
Some years ago we published our results on the microwave assisted aza-Cope rearrangement of N-allylanilines. This reaction yields ortho-allyl anilines, which are interesting substrates for the preparation of different bicyclic heterocycles. But as you will see in this paper by Bugarin et al. (University of Texas, TX, USA), you can manipulate them into a broader range of internal alkenes with a functional group in the terminal carbon.
The two-step protocol involves a bromination of the double bond with molecular Br2, carried out in CHCl3 at rt, followed by treatment of the dibromo compound with a suitable nucleophile, using DBU as base in DMSO. In this second step the double bond is regenerated, but in the internal position, and the nucleophile is attached to the end. And the beauty of the protocol is the selection of nucleophiles. Using AcOH yields allylic acetates. Other acids, give the corresponding esters (examples include benzoates, cinnamates and myristates). Compounds with active methylene groups (malonates, 2-nitroacetates…) give quite interesting building blocks. Phthalimide gives the well known intermediate of a Gabriel amine synthesis. And thioacids give thioesters. Not many examples with heterocycles, but who knows…
Org. Lett. 2015, Article ASAP.
See: 10.1021/acs.orglett.5b00862