Room-Temperature and Phosphine-Free Palladium-Catalyzed Direct C-2 Arylation of Indoles
Direct C–H functionalization is an attractive way to increase molecular diversity. A new development allows the C-2 arylation of indoles using a room-temperature Heck reaction.
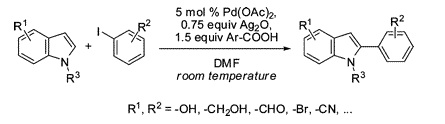
Direct C–H functionalization is very attractive for a number of reasons. No need of halides, boronics, stannanes, or others, and no need for directing groups. Just your molecule… and a suitable method to put the desired group in the correct C–H bond. The research group of Larrosa (London, UK) has published a paper reporting improved conditions for the direct C-2 arylation of indoles. The method relies in the use of Pd(OAc)2 as the palladium source. The drawback of the high temperatures commonly required for this transformation is overcome through the use of an additive, a silver salt that generates a highly electrophilic palladium complex that can react at room temperature with the indole to yield the desired product. In an additional tour de force, the silver salt is generated in situ from Ag2O and ortho-nitrobenzoic acid. Interestingly, no phosphine or ligand is used. The final conditions allow the introduction of an aryl group at room temperature in yields from good to excellent. The conditions are compatible with sensitive groups.
J. Am. Chem. Soc., 2008, 130 (10), pp. 2926–2927. See: 10.1021/ja710731a