In the last years there has been an increasing number of chiral New Chemical Entities (NCEs) entering the pipeline of the pharmaceutical companies. Most of these NCEs contain only one asymmetric center, so two enantiomers of the drug are theoretically available. It is well known that sometimes only one of the enantiomers have a therapeutic effect while the other may have an entirely different effect, no effect at all, or even have undesired side effects. The continuous interest in developing pure chiral drugs boosts the development of new asymmetric synthesis methods, but sometimes the focus on this development clouds or even neglects common methods.
Chiral Drugs
This neglecting comes from a number of reasons, including scientific and marketing components, but sometimes also ignorance of fine points in the development of commercial drugs. A known trend in the last years has been marketing single enantiomers of known racemic mixtures, with great sales successes like escitalopram (1) and esomeprazole (2); but from this point the idea of asymmetric synthesis being a key, essential component of the drug discovery process from the beginning has extended. However, three options are available for obtaining the desired enantiomer of a compound: First, enantioselective synthesis of the compound; second, chiral preparative HPLC; and third, a classic racemic resolution of the mixture.
Though from the economic point of view the best solution can be the asymmetric synthesis one, development of asymmetric methods makes usually sense for the development stages of the project, where cost is everything, it takes time and effort. A big emphasis has been done on development of asymmetric homogenous catalysts and biocatalysts (i.e., enzymes), but for the preclinical trials, batches of hundreds of grams of enantiomerically pure compound are needed quickly, a problem which development faces every day.
Some additional points must be considered when balancing an asymmetric synthesis vs. a racemic synthesis:
1. It can be better to try both enantiomers first. According to the FDA guidances, ‘In general, it is more important to evaluate both enantiomers clinically and consider developing only one when both enantiomers are pharmacologically active but differ significantly in potency, specificity, or maximum effect, than when one isomer is essentially inert.’ (3)
2. From the regulatory point of view both enantiomers should be tested: ‘To evaluate the pharmacokinetics of a single enantiomer or mixture of enantiomers, manufacturers should develop quantitative assays for individual enantiomers in in vivo samples early in drug development. This will allow assessment of the potential for interconversion and the absorption, distribution, biotransformation, and excretion (ADBE) profile of the individual isomers.'(3)
3. It is not always possible to prepare both enantiomers with an asymmetric synthesis, specially in biocatalysis. I.e., enzyme X renders wonderfully the desired enantiomer, but there is no anti-enzyme X (or another different enzyme) which renders the other enantiomer.
4. Development of simpler methods, perceived as less challenging and/or even technologically obsolete can serve to block possible challenges from competitors and extend the lifetime of a drug. (4)
Chiral preparative HPCL is an excellent solution on the preclinical stages, since it renders both pure enantiomers for initial activity and toxicology assays, but is usually too expensive to being used in manufacturing, though there are exceptions like escitalopram. (5)
Finally, the racemic resolution process uses the racemic mixture as raw material for a crystallization process where a chiral adjuvant is introduced, a salt of the desired enantiomer separated and the single enantiomer released. Though this process gives away at least half of the raw material, it is developed quickly, renders the product with good chemical and optical purity and it is cheap. It is a popular method and many drugs are prepared through a racemic resolution process.
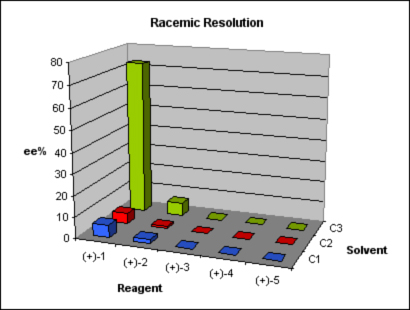
As study case, one of our clients approached us with a request to develop a racemic resolution method for a promising preclinical candidate. Initial assays showed that the enantiomer S presented better activity than the R. Chirality came from a dimethylamino moiety introduced by means of an aminative reduction of a ketone. We were provided with the details of a straightforward route in two steps, considering that the needed building block for the coupling was also supplied. A method for the enantioselective synthesis of the compound was being developed, but to cover intellectual property claims and allow a faster supplying of bigger batches of enantiomerically pure compound for the upcoming trials a classic racemic resolution was required. We applied Design of Experiment (DoE) principles, parallelization techniques and analytical skills to the problem and offered to our client in eight weeks not one, but two solutions, with a broader range of conditions to make a choice.
As conclusion, each alternative has advantages and disadvantages. Not only cost must be considered, but time pressure, technological feasibility and coverage of important intelectual property issues when the product faces the regulatory phases.
(1) Escitalopram (Lexapro/Cipralex) is the single enantiomer of Citalopram (Celexa/Cipramil).
(2) Esomeprazole (Nexium) is the single enantiomer of Omeprazole (a generic now).
(3) FDA: Development of New Stereoisomeric Drugs (see FDA site)
(4) Drug and Therapeutics Bulletin 2006, 44, 73-77. (5) Manufactured by Lundbeck using Simulated Moving Bed Chromatography.