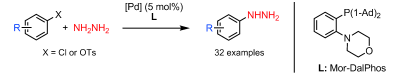Palladium-Catalyzed Cross-Coupling of Aryl Chlorides and Tosylates with Hydrazine
Finally, the direct introduction of hydrazine through a Buchwald-Hartwig coupling.
The introduction of a nitrogen atom via an organometallic coupling has been one of the most active fields in Organic Synthesis in recent years. Two names stand out: Buchwald and Hartwig. The so-called palladium catalyzed amination of aromatic compounds is therefore known as Buchwald-Hartwig reaction. Along the years, many conditions have been developed, allowing the coupling of a broad range of amines and amides with haloaromatic compounds and other suitable partners, as triflates and tosylates. Hydrazine can be introduced using this reaction, but only if you mask it: one of the most used approaches is the use of a hydrazine surrogate with attenuated reactivity, benzophenone hydrazone. Once the surrogate is coupled you must deprotect the hydrazone following a couple of protocols. But those protocols do not work all the time, and of course you are deprotecting. Getting rid of this or other protecting groups and using hydrazine directly would be very welcomed.
That is what Stradiotto et al. (Dalhousie University, Canada) have published now. After their initial success using NH3 as nitrogen source in the Buchwald-Hartwig reaction, they have tackled the hydrazine problem and developed a working protocol. The protocol involves heating a phenyl chloride or phenyl tosylate, hydrazine monohydrate (ratio 1:2), [{Pd(cinnamyl)Cl}2] (3-5 mol% usually), the ligand (5-8 mol%) and NaOtBu (2 eq.) in toluene at 90 °C for 30-60 minutes. The trick? The ligand must be Mor-Dal-Phos, which is not commercially available. The method works with 22 different aryl chlorides, including 4-chloropyridine which is chosen because hydrazine can react with the 2- and 4- isomers through other mechanism, and 7 tosylates. Once you put your hands on the phosphine, really worth to try it !!
Angew. Chem. Int. Ed., 2010, 49 (46), pp 8686–8690. See: 10.1002/anie.201003764