Formation of ArF from LPdAr(F): Catalytic Conversion of Aryl Triflates to Aryl Fluorides
A new method for the fluorination of compounds via palladium catalysis.
Incorporation of fluorine into aromatic organic compounds is one of those fields of permanent interest. Fluorine is important for many reasons, including their ability to increase metabolic stability, solubility, and bioavailability. About 25% of all drugs on the market contains at least one fluorine atom, and this ratio will probably increase in the near future. However, getting fluorine atoms into aromatic organic compounds has remained extremely challenging. Up to now.
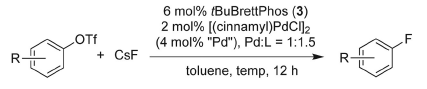
Now, for the first time, a team of chemists led by Buchwald (Boston, USA) has developed conditions to introduce fluorine atoms by palladium catalysis. This reaction is probably one of those Holy Grails in organic synthesis and, as Buchwald says, ‘it has been one of the missing reactions that people in the pharmaceutical industry have been asking about quite a bit’.
The method is still far from perfect, but it seems that the use of aryl triflates as substrates and a reagent system comprising a palladium source, tBuBrettPhos and CsF in toluene under heating, yields fluorinated compounds in good yields. The scope reported is broad, but it can be greatly enhanced. The main problem of the reaction is the formation of small amounts of regioisomers. In any case, a real breakthrough.
Science, Published Online August 13, 2009. See: 10.1126/science.1178239