Concise Synthesis of 2-Amino-4(3H)-quinazolinones from Simple (Hetero)aromatic Amines
Short methods for the preparation of nitrogen heterocycles are always welcome. In this case, a new synthesis for 2-alkylaminoquinazolinones is described.
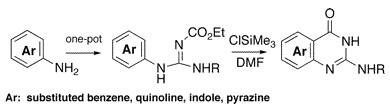
Nitrogen heterocycles are the daily bread for medicinal chemists. Many methods are available for constructing a given structure, but sometimes the available chemistry lacks versatility, it is too substrate-dependent or needs scarce specific starting products. The methods for 2-alkylaminoquinazolinones suffer from such problems. The research group of Demeunynck (Grenoble, France) has published a short, two-step method for constructing this ring system from available aromatic amines and ethoxycarbonyl isothiocyanate, also commercially available. The method involves the reaction of a starting amine with isocyanate to give an intermediate guanidine, which is subsequently cyclized using ClTMS. The final products precipitate and can be purified without chromatography. Examples with anilines, carbocyclic-fused anilines, indoles, pyrazoles and others are described.
J. Org. Chem., ASAP See: 10.1021/jo7026883