A New Paradigm for Carbon–Carbon Bond Formation: Aerobic, Copper-Templated Cross-Coupling
We are used to carry out reactions to yield a product. How about a reaction that yields two different products at the same time?
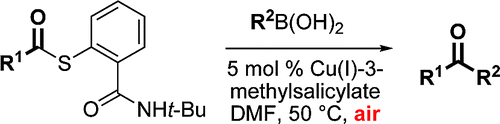
The formation of arylketones is an old reaction commonly done using the Friedel–Crafts reaction. The development of organometallic reactions has expanded the available methods to prepare these ketones avoiding the use of AlCl3 or other Lewis acids. The group of Liebeskind (Atlanta, GA, USA) has now published a new striking method that does not only yield the ketone, but also an S-arylated compound. The method requires the preparation of S-acyl-NHt-Bu thiosalicylamides, which are reacted with two equivalents of a boronic acid in the presence of air and CuI-3-methylsalicylate. Though the acyl-transfer part and the catalyst need to be prepared, the reaction shows potential to yield efficiently a broad range of ketones using available boronic acids and also the corresponding S-aryl-NHt-Bu thiosalicylamides.
J. Am. Chem. Soc., 2007, 129 (51), pp. 15734–15735. See: 10.1021/ja074931n