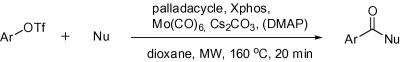
Microwave-Promoted Aminocarbonylation of Aryl Triflates Using Mo(CO)6 as a Solid CO Source
A new entry in carbonylation reactions, this time with an alternative to CO.
In our last issue, we presented two papers published by the group of Buchwald on the room-temperature carbonylation of aryl chlorides and bromides, which addressed some drawbacks of the common carbonylation protocols: high pressure and harsh conditions.
The work by Larhed et al. (Uppsala, Sweden) that we present here addresses other drawbacks of the current protocols. More specifically, it deals with the need for using CO gas, which is toxic, and long reaction times.
The protocol presented in the paper is based on the use of Mo(CO)6 and microwaves but, in contrast to the methods of Buchwald, the substrates of election are triflates. In a typical procedure, the triflate, a nucleophile (amine, alcohol or sulfonamide), Hermann’s palladacycle (2.5 mol%), Xphos (7.5 mol%), Cs2CO3, and piperidine in dioxane are heated under microwave irradiation at 160 °C for 20 min. Products are obtained in yields from good to excellent.
Both the Hermann’s palladacycle catalyst and the ligand are commercially available from common commercial suppliers. Additional points of interest are that first, the reaction with bulky amines can be improved by using DMAP and second, one example of a tosylate as substrate is provided, with a 55% yield. This new method sounds promising for application in parallel microwave reactors, specially if it can be further expanded to include aryl halides.
Tetrahedron Lett., 2008, 49 (42), pp 6115–6118. See: 10.1016/j.tetlet.2008.08.014