Copper-Catalyzed Domino Annulation Approaches to the Synthesis of Benzoxazoles under Microwave-Accelerated and Conventional Thermal Conditions
Sequential one-pot formation of two bonds is an attractive way to make things. See here an example applied to benzoxazoles.
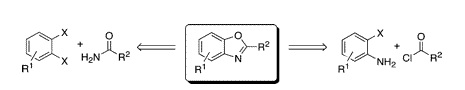
Benzoxazoles are widespread among medicinal chemistry targets. The usual synthesis of benzoxazoles uses as raw materials a 2-aminophenol and an acid or aldehyde. Obviously, this narrows the scope of starting materials available for the synthesis of benzoxazoles, and methods using a new pool of compounds are thus always welcome. The group of Batey (Toronto, Canada) had published in 2006 a first work in this direction, and they have presented now a paper with two domino approaches for the synthesis of benzoxazoles. The first approach uses 1,2-dihalobenzenes and primary amides, with a first intermolecular copper-catalyzed C–N cross-coupling, followed by an intramolecular copper-catalyzed C–O cross-coupling. However, this approach is limited both by the availability of 1,2-dihaloarenes and regioselectivity problems. The second approach, and clearly the winning one, starts from 2-haloanilines and acyl chlorides, building the oxazole ring with an intermolecular N-acylation, followed by intramolecular copper-catalyzed C–O cross-coupling using CuI, Cs2CO3, and 1,10-phenantroline in DME under reflux. There is plenty of commercial 2-haloanilines and acyl chlorides, which makes this approach much more interesting. Although the original method involves extended times, a microwave-assisted methodology has also been developed, with reaction times of 15 min and good yields. This protocol is used for the automated preparation of a library of 24 benzoxazoles with an average yield of 72%.
J. Org. Chem., 2008, 73 (9), pp 3452–3459. See: 10.1021/jo702145d