Synthesis of Unnatural Amino Acid Derivatives via Palladium-catalyzed 1,4-Addition of Boronic Acids
Take advantage of many boronic acids to get unnatural aminoacids.
Synthesis of unnatural amino acids is key to the preparation of therapeutic peptides. Many methods are available, but only a few make use of the last developments in organometallic couplings. Boronic acids are widely available, so any method that can take advantage of these starting materials for producing new unnatural amino acids is highly interesting.
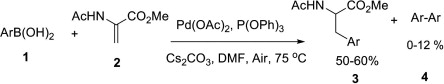
This paper by Natarajan (Eppley Institute for Cancer Research, NE, USA) makes use of recent developments in the conditions for the 1,4-addition of arylboronic acids to a,b-unsaturated compounds. The key modification for the success of this reaction is the use of a palladium-phosphite system instead the usual palladium-phosphine. The reaction can be carried out under oxygen atmosphere (in fact, it must be carried out under oxygen atmosphere) and allows the preparation of amino acids from aryl- and heteroarylboronic acids and methyl-2-acetamidoacrylate.
In the usual protocol the arylboronic acid (1.3 equiv), methyl-2-acetamido acrylate, Pd(OAc)2 (6 mol%), P(OPh)3 (6 mol%) and Cs2CO3 are solved in DMF and heated to 75 °C for 3-5 h. Yields are good, and the main subproduct of the reaction is the homocoupling product Ar-Ar. Something to consider the next time you look for a different amino acid.
Tetrahedron Lett., 2010, 51 (19), Pages 2655-2656. See: 10.1016/j.tetlet.2010.03.034