Hydride as a Leaving Group in the Reaction of Pinacolborane with Halides under Ambient Grignard and Barbier Conditions. One-Pot Synthesis of Alkyl, Aryl, Heteroaryl, Vinyl, and Allyl Pinacolboronic Esters
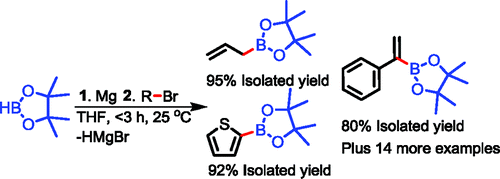
Protocol for the preparation of pinacolboronates from magnesium reagents.
What a title! Well, if you leave aside the ambient, Grignard, Barbier and other terms, this paper is about boronic esters. Pinacolboronic esters. Singaram (University of California, CA, USA), describes a method for the coupling of magnesium reagents with pinacolborane. This reagent is half the bis(pinacolborane), the most used source of boron when preparing boronates using the Suzuki-Miyaura reaction. But there half of the boron source mass is lost, while here you have an elegant atom economy, since only one hydrogen is lost. The paper contains a lot of mechanistic info, kinetics, NMR experiments and so, but for those interested in the results, they describe 10 examples of boronates with Grignard reagents, including some alkyl boronates (do you know that you can alkylate arylamines with that using the Chan-Lam reaction?), 12 examples using the Barbier protocol, including a couple of heterocyclic substrates (thiophenes) and finally 5 examples of allylboronates, all them with excellent yields. So another tool for preparing boronates!
J. Org. Chem., 2011, 76 (23), pp 9602?9610. See: 10.1021/jo201093u