Microwave-enhanced and Ligand-free Copper-catalyzed Cyanation of Aryl Halides with K4[Fe(CN)6] in Water
A microwave-enhanced cyanation in water using an iron-cyanide complex.
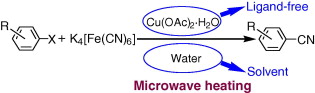
This paper by Wang (Henan University, Henan, China) contains some interesting points for both medicinal chemists and process development chemists. The work is centered in the development of an economical and environmentally benign method for the copper-catalyzed cyanation of aryl halides. The method uses Cu(OAc)2·H2O as the catalyst, which is not so uncommon for a cyanation method. However, the cyanide source is the non-toxic complex K4[Fe(CN)6]. The method makes use of microwave technology, so in a typical procedure, a mixture of an aryl halide, Cu(OAc)2·H2O, K4[Fe(CN)6], and TBAB in water is heated under microwave irradiation at 140 °C for 50 min. The time is incredibly long for a microwave method, but yields are usually excellent. Twenty-nine examples are reported, including aryliodides and bromides, with a couple of heterocycles. In the case of bromides, KI and DMEDA are used as additives. The method gives excellent results even in some challenging cases, such as ortho-substituted substrates.
Tetrahedron Lett., 2009, 50 (32), pp 4595–4597. See: 10.1016/j.tetlet.2009.05.073