Enhanced reactivity of silver- and gold-catalysed hydrogenations using silver(I) salts
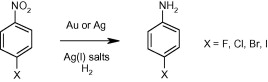
Using gold and silver to hydrogenate halonitroaromatics.
If you have ever tried the hydrogenation of an aromatic halonitrobenzene you probably know that it is not always an easy reaction. Besides the common issues of reducing a nitrocompound, the halogen adds a challenging group which is sometimes wiped out in the hydrogenation, leaving a nice mixture of halogenated and dehalogenated compound, that sometimes simply cannot be separated. Is common knowledge that Palladium catalysts give more dehalogenation than Platinum catalysts, but as usual, nothing is so simple. There are around some catalysts that work wonderfully for the hydrogenation of halonitrobenzenes and halonitroheteroaromatics, but here we have a nice addition from Johnson Matthey.
The paper, published by Fussell et al. (Pfizer, UK) studies briefly the use of Silver and Gold catalysts on an Al2O3 support. After thorough experimentation, they have found that both 5% Ag/Al2O3 and 5% Au/Al2O3 catalysts avoid the competing dehalogenation of the substrate. However, in order to accelerate the reaction an additive is needed, and the best are silver salts like AgOAc and Ag2CO3. Chloronitrobenzenes are the best substrates for the reaction conditions (with a hydrogen pressure of 150 psi!), though the only example with bromine stands fine, but with lower conversion. Only the iodonitrobenzene does not survive, nothing unexpected here. Next time you need to reduce one of these substrates, make a call to your JM agent!
J. Am. Chem. Soc., 2010, 132 (24), pp 8270–8272. See: 10.1016/j.tetlet.2010.07.143