C–H Methylation of Heteroarenes Inspired by Radical SAM Methyl Transferase
A commercial reagent that you can use to introduce methyl groups directly into your heterocycles.
Some years ago, I had some laughs with a friend doing his PhD one bench away. He was working in HIV proteases, and he told me that talking to the computational chemist, the poor guy marked an atom in the molecule and the following conversation took place:
— ‘It would be really interesting to put a methyl group there and check the activity. How much time do you need?’
— ‘Oh, about 6 months or so and you will have your results’.
— ‘Six Months!? You need six months just to put a methyl!?’
— ‘Well, you know, I have to introduce the methyl from the beginning. This is not like Lego, you can’t simply replace a hydrogen with a methyl.’
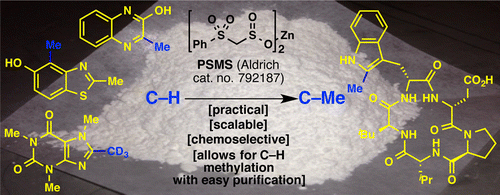
Well, Organic Synthesis is definitely not like Lego, but some things are changing. The Baran group (Scripps, CA, USA) has published and licensed its work on the development of a new methylation reagent, inspired in the Radical SAM Methyl Transferase. This is an enzyme that Mother Nature uses to methylate things like nucleotides, aminoacides and so, using S-adenosylmethione (SAM) as methyl source. Very recently we have published a short entry with a recent (hem!) review about the Magic Methyl concept.
In summary, they developed a reagent called zinc bis(phenylsulfonylmethanesulfinate) (PSMS) which is used in combination with TBHP in PhCF3/H2O. The reaction can be done under oxygen atmosphere, at room temperature, and the reagent is user friendly. You can purchase it from Aldrich (you are welcome, Merck). In fact, the reaction product is not the methylated compound, but a (phenylsulphonyl)methyl product that must be transformed into the corresponding methylated compound using three different methods: Mg in MeOH, SmI2 in THF/H2O or Raney-Ni in EtOH.
Obviously, the reagent relies in the C-H activation of some appropriate C-H bonds in heterocycles. So far, they have tried things like caffeine, pyrroles, imidazoles, pyrimidines, pyridazines, indoles and many others (though NOT pyrazoles, grrrrr). So what are you waiting for?
J. Am. Chem. Soc. 2014, 136(13), pp 4853-4856.
See: 10.1021/ja5007838