Synthesis of 2-Substituted Pyrazolo[1,5-a]pyridines through Cascade Direct Alkenylation/Cyclization Reactions
Synthesis of fused pyrazoles by coupling with an activated C-H.
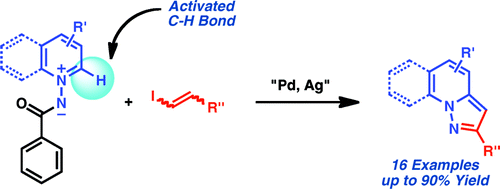
If you follow the medicinal chemistry literature, you will note that scaffolds, as with many other things, follow fashion trends. And some scaffolds, quite simply, are in fashion, and fused nitrogen heterocycles are always «in style». Pyrazolo[1,5-a]pyridines have certain interest as adenosine A1 antagonists and are hard to find. The usual route leading to these compounds yields structures with substitution in the position 3, but to access structures with substituents in the position 2, the only available routes are lengthy and limited.
The route published by Charette (Montreal, Canada) addresses this problem and relies in the use of a N-iminopyridinium ylide, which are versatile scaffolds for the preparation of pyridine derivatives. The C-H bond adjacent to the nitrogen atom is activated towards a palladium-catalyzed alkenylation. This, combined with the serendipious discovery that using Ag2CO3 as a base during the coupling triggers a cyclization of the system, are the fundamentals of the method.
In a typical reaction the pyridinium ylide is mixed with P(4-MeOPh)3 (15 mol%), PdBr2 (5 mol%) and AgOBz (300 mol%). The alkenyl iodide derivative (100 mol%) is added diluted in 1,4-dioxane and the reaction mixture is irradiated at 125 °C for 16 hours. Though no modifications are tested on the pyridine moiety, the alkenyl iodide admits many variations.
Org. Lett., 2010, 12 (3), pp 516–519. See: 10.1021/ol902710f