Synthesis of Fully Substituted Pyrazoles via Regio- and Chemoselective Metalations
By third time in a row, pyrazoles get a slot on the top.
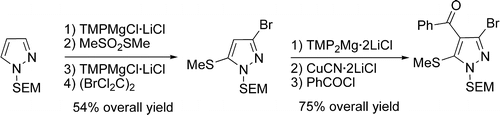
In the two previous issues of our newsletter, we included as many papers on the synthesis of pyrazoles. Both contained the concept ‘regioselective’ in the title, something that reflects the true limitation of the older synthetic methods. This time the paper comes from the group of Knochel (Munich, Germany). I must admit that I am not very affectionate to metallation methods, because you usually have to work at low temperatures and stability of metallated compounds is usually an issue. In this case, though exotic, the organometallic bases used do not require extreme conditions. The paper focuses on the use of Li/Mg mixed bases, TMPMgCl·LiCl and the more reactive TMP2Mg·2LiCl (where TMP is 2,2,6,6-tetramethylpiperidyl).
As in the paper of Sames presented in the previous issue, the starting product is a SEM-protected pyrazole, or alternatively, 1-methylpyrazole. A first metallation with TMPMgCl·LiCl allows functionalization at position 5, which in turn, by trapping or trapping/Negishi steps, furnishes 5-functionalized pyrazoles. A subsequent deprotonation at position 3 is readily achieved by adding TMPMgCl·LiCl. Again, suitable trapping yields 3,5-difunctionalized pyrazoles. The third and last metallation at position 4 requires the more reactive base TMP2Mg·2LiCl, but further trapping gives the final 3,4,5-trifunctionalized pyrazoles.
The striking thing about these bases is the temperature required: all metallations are carried out at -15 °C or -20 °C. That gives you an idea about how smooth these reactions are. A broad range of electrophiles is used in the trapping step, including -F, Cl, Br, -CN, -CHO, -COPh, and allyl sources. As an example, Tebufenpyrad is synthesized. Unfortunately, the bases are not commercial and must be prepared according to literature procedures.
Org. Lett., 2009, 11 (15), pp 3326–3329 See: 10.1021/ol901208d
