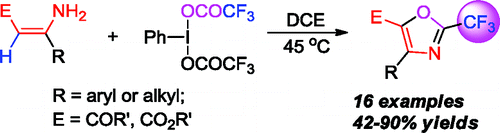
Synthesis of 2-(Trifluoromethyl)oxazoles from ß-Monosubstituted Enamines via PhI(OCOCF3)2-Mediated Trifluoroacetoxylation and Cyclization
Synthesis of trifluoromethylated oxazoles from enamines and PIFA.
If this goes on, we will have to open a section in the newsletter under the label ‘CF3’. This time is a paper by Du and Zhao (Tianjin University, China). They report a nice reaction using enamines and phenyliodine bis(trifluoroacetate) (PIFA) to prepare trisubstituted oxazoles with a CF3 moiety in the C-2. 16 examples are included, with different aryl and alkyl substituents in the C-4 and C-5 positions.
J. Org. Chem., Article ASAP. See: 10.1021/jo202070h