A Simple and Convenient One-pot Method for the Preparation of Heteroaryl-2-imidazoles from Nitriles
Some chemists say that old chemistry is the best. This is true, specially when you improve it to modern standards to prepare imidazoles.
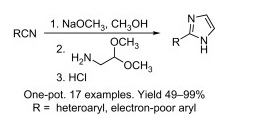
Imidazoles are important in the synthesis of pharmacologically active compounds, but those with a single heterocyclic substituent in the 2-position are not easy to prepare. Chemists from AMRI and CGI Pharmaceuticals have retaken an old method an improved it to modern standards. The key intermediates are amidines, which are useful synthetic intermediates for the preparation of different heterocycles. This remarkable synthesis uses commercially available nitriles as starting materials, which are transformed into the corresponding amidines under basic conditions. The amidines are not isolated, but treated with ethyl or methyl aminoacetal. Addition of an acid causes the intermediate to cyclize yielding the expected imidazoles in excellent yield. The whole procedure can be done in one-pot, is easily scaleable, and does not require chromatography purification.
Tetrahedron, 2008, 64 (4), pp 645–651. See: 10.1016/j.tet.2007.11.009