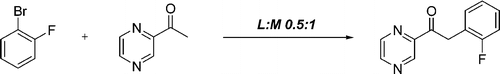Mild and General Method for the a-Arylation of Heteroaromatic Ketones
With heterocycles, sometimes it is worth to check the ligand to catalyst ratio.
The a-arylation reaction is an attractive method for the rapid construction of C-C bonds adjacent to a carbonyl group. Though a great effort has been devoted to this topic, most of the methods available are focused on the a-arylation of normal aromatic ketones, not heteroaromatic substrates. All medicinal chemists would love to have at hand an improved , more flexible and powerful method, than can be applied to azines.
This paper by Desai (Bristol-Myers Squibb, NJ, USA) is an advance in that direction. They aim to develop a mild method for the monoarylation of a methyl heteroaromatic ketones. And along the way they have discovered that the ligand to metal ratio is critical, so they have compiled a list of different azines and other heteroaromatic methyl ketones with their optimal ligand to metal ratios. For example, different acetyl pyrimidines have L:M ratios of 0.5 up to 1.5, depending on the position. The protocol involves heating the ketone and the aryl bromide or chloride in the presence of Pd(OAc)2 (4 mol%), Xantphos and K3PO4 (2.8 equiv) in THF:1,4-dioxane:toluene at 100 °C for 18 h. Yields are usually excellent, though the reaction times are long. No comment is made on microwave heating, and unfortunately all solvents used are almost transparent to microwave irradiation, so unless the catalyst and base provide enough ‘polarization’ of the medium, not a big enhancement would be seen. The extensive work includes pyridines, pyrimidines, indoles, furanes and even dihydroisoquinolines which are usually constructed through the old Bischler-Napieralski cyclization. A detailed revision of the paper is higly recommended !
Org. Lett., 2010, 12 (5), pp 1032–1035. See: 10.1021/ol1000318