Zn-Mediated, Pd-Catalyzed Cross-Couplings in Water at Room Temperature Without Prior Formation of Organozinc Reagents
Easy and wet protocol for the Negishi reaction.
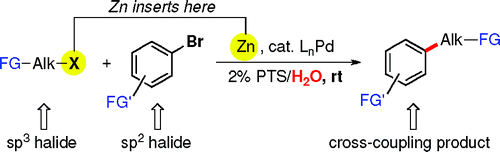
The palladium-catalyzed cross-coupling of organozinc reagents has attracted increasing attention in the last years for several reasons. It is one of the few couplings with the ability to render products from sp2–sp3 couplings, instead of the usual sp2–sp2 couplings in many other reactions. However, preformation of an organozinc halide was mandatory. Until now.
The group of Lipshutz (California, USA) has published a very interesting paper reporting a new protocol to perform a Negishi coupling. The protocol does not need a zinc halide as starting material, just the halogenated compounds. In a typical procedure, zinc powder and PdCl2(Amphos)2 are added to a 2% weight solution of PTS (polyoxyethanyl-a-tocopheryl sebacate) in water. TMEDA is added at rt, followed by addition of the primary alkyl iodide or bromide and the aryl bromide. Products are obtained in yields from good to excellent. The protocol is striking for three reasons. First, the zinc halide is not preformed, a very important point that the authors stress even in the title of the paper. Second, you just have to mix everything at room temperature and stir. No need to heat, microwave irradiate, or else. And third, the reaction can be done in water, a feature acquired through the use of PTS. Thus, this protocol has many advantages and a very green future. By the way, everything your need to give it a try is commercially available.
J. Am. Chem. Soc., 2009, 131 (43), pp 15592–15593. See: 10.1021/ja906803t