PyDipSi: A General and Easily Modifiable/Traceless Si-Tethered Directing Group for C-H Acyloxylation of Arenes
Remote functionalization using a silicon-tethered directing group.
I read once a book stating that ‘when trying to find a solution for your problem you sometimes search the literature like former president Nixon once perused the Constitution: looking for a way out’. Well, sometimes I have the same feeling when searching the catalogs of the commercial suppliers looking for the product with the proper substitution pattern: please, please…
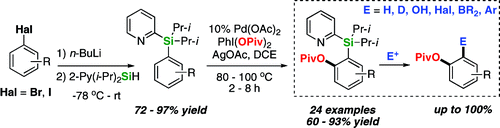
So new methods to further functionalize a product are always interesting to me. The method we present here is based in recents developments in C-H activation. To summarize, it trades a halogen (bromine or iodine) by the directing group PyDipSiH. This group, posesing a pyridine ring, allows the activation and acetoxylation of the adjacent C-H bond using Pd(II) chemistry. Very interestingly, the reaction is quite regioselective; i.e., when using the 3-methylbromobenzene, the C-H activated is located in para to the methyl group. Similar regiochemistries have been mapped with other substrates, like naphthalenes. Once the functionalization is done, the directing group can be safely transformed into other useful groups like iodide, hydroxy, or boronate; it can be used for a coupling using Palladium chemistry or removed to give hydrogen or deuterium. The introduction of the pivaloxy group is an additional bonus of this method, since it can be used as a valuable synthetic group.
One of the drawbacks of the protocol is that the PyDipSiH is not commercial and must be prepared from 2-bromopyridine by metallation with n-BuLi and trapping with i-Pr2SiHCl. The preparation is described in the supplementary info of the paper in a 170 mmol scale with a 91% yield. The installation of the PyDipSiH group into the substrate is done by metallation/trapping. Once accomplished, the acetoxylation can be carried out.
In a typical procedure, the 2-(diisopropyl(aryl)silyl)pyridine (0.5 mmol), Pd(OAc)2 (5 mol%), PhI(OPiv)2 or PhI(OAc)2 (200 mol%) and AgOAc (1 equiv.) under N2 atmosphere are dissolved in DCE or butyronitrile and heated at 80–100 °C for 2–8 h until the reaction is complete. Yields are excellent, and the reaction is compatible with many functional groups like ethers, halides, esters, amides and boronates. Maybe the reaction is not suitable for complex substrates, but it should be a nice addition to prepare starting materials and building blocks.
J. Am. Chem. Soc., 2010, 132 (24), pp 8270–8272. See: 10.1021/ja1033167