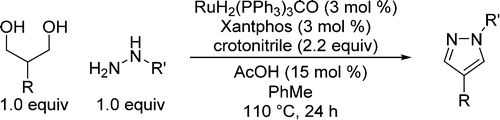
Synthesis of Pyrazoles from 1,3-Diols via Hydrogen Transfer Catalysis
A method for the synthesis of 1,4-disubstituted pyrazoles using ruthenium-catalyzed hydrogen
transfer.
Good news: Pfizer is still doing chemistry. And this paper is the proof. Schmitt et. al (Pfizer, CT, USA) have worked in a new method to prepare 4-alkyl pyrazoles without resorting to the usual condensation of hydrazines with 1,3-dicarbonyl compounds. As they state in the introduction of the paper, though there are plenty of methods to prepare pyrazoles, all of them have limitations: lack of regioselectivity, problems with the precursors, no access to specific substitution patterns, etc.
To avoid one of this shortcomings, the unstability of some 1,3-dicarbonyl precursors, they have applied recent advances in something called hydrogen transfer, which is basically the in situ dehydrogenation of alcohols catalyzed by metals (yes, there is a catalyst here). So the hydrogen transfer avoids the synthesis of a potentially unstable carbonyl intermediate by oxidation: that is done at the same time you carry out the condensation with the hydrazine component. After some experiments, they found that RuH2(PPh3)3CO and Xantphos do the trick. Some additives are needed (crotonitrile and acetic acid), but all considered, not a bad reaction. They explore both the hydrazine and alcohol influence in the reaction and you have quite interesting examples in the schemes (though they could use a neat table instead those schemes). The reaction works also in one gram scale, so it seems to have the potential to be used as initial step in the synthesis of a pyrazoles library.
Or. Lett. 2015, 17(6), pp 1405-1408.
See: 10.1021/acs.orglett.5b00266