The ring opening of epoxides is one of those things that you learn during your general organic chemistry courses. Look back and recall the Markovnikov’s and Anti-Markovnikov’s rules, Lewis acids, and SN2 mechanisms.
Well, that basic knowledge is something you really have to use when designing a synthetic route, because you need to predict what will happen to the epoxide during the reaction. Will we obtain a product through ring opening at this position? Or will the epoxide open the other way? Very recently, we faced exactly this problem during the synthesis of a metabolite, where the addition of an amine hydrochloride was affording different results depending on whether we added a base and the type of said base.
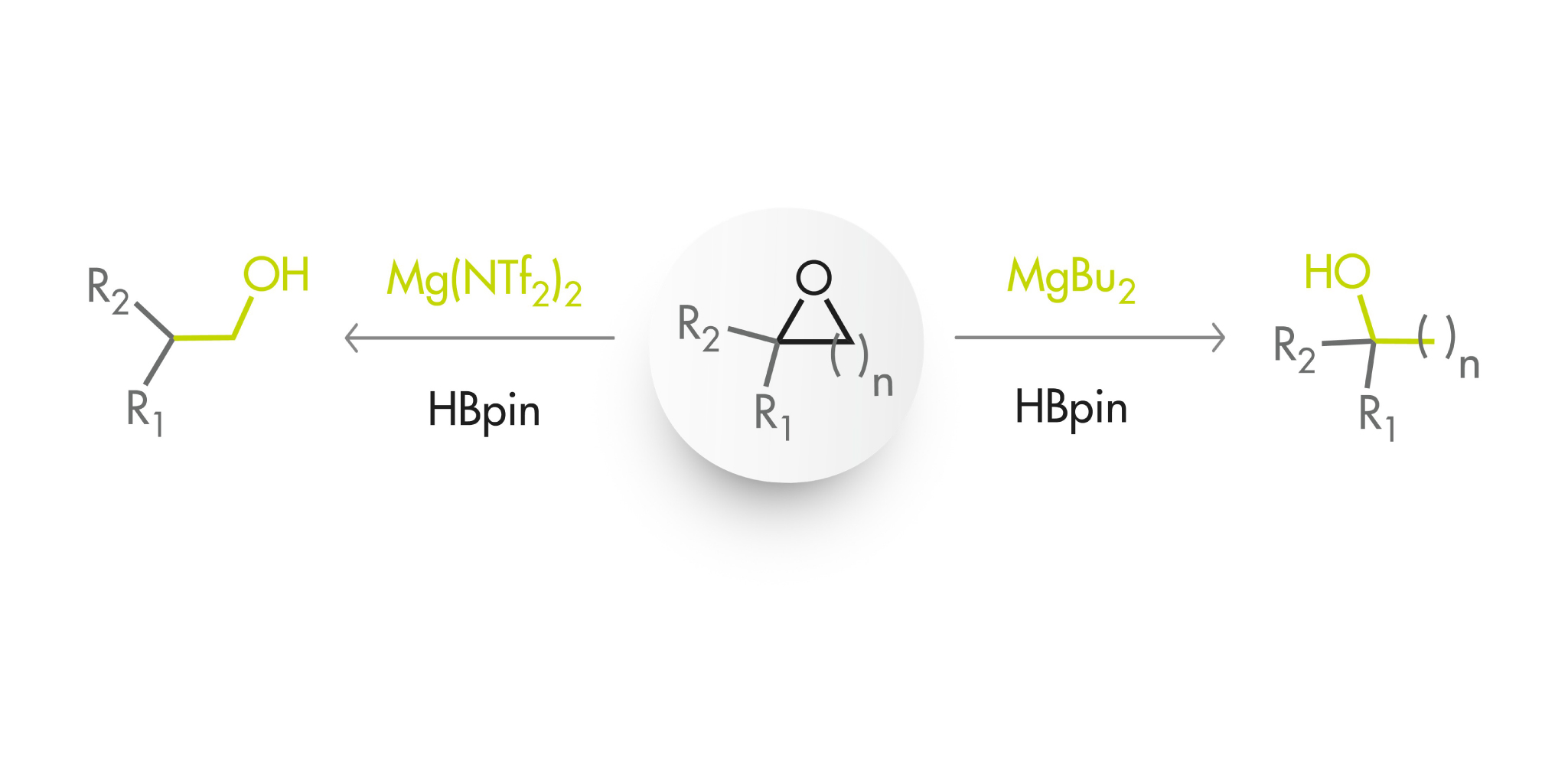
However, this was a case of stoichometric ring opening and sometimes what you need to perform is a catalytic ring opening, for example, when you are trying to reduce an epoxide to obtain an alcohol. This work by Cavallo, Rueping and collaborators focuses exactly on that problem. They tried commercially available dialkyl magnesium compounds to perform a catalyzed reaction with HBpin as the reducing agent and found that 5% of MgBu2 at 40 °C in THF is a good starting point. In fact, the results were much better with complex substrates than with the model substrate.
Encouraged by these results and applying their experience in the reduction of ketones with a similar approach, they decided to replace MgBu2 by Mg(NTf2)2 (also commercially available) and evaluate their effect on both the activity and selectivity. And, surprise! The latter catalyst switches the regiochemistry the other way, which they obviously attribute to a different HBpin activation and ring-opening mechanism. The article includes some experiments and DFT calculations to address that question.
So that is that: with the epoxide at hand, MgBu2-HBpin produces one of the alcohols and Mg(NTf2)2–HBpin the other one. In summary, a neat method that works!
Regiodivergent Hydroborative Ring Opening of Epoxides via Selective C–O Bond Activation J. Am. Chem. Soc. 2020, 142(33), 14286–14294. See: 10.1021/jacs.0c05917