Easy, chemoselective, safe, non-toxic, scalable, cheap… All these words give a pretty nice summary of the main goals that all chemists seek when they want to design the perfect chemical process.
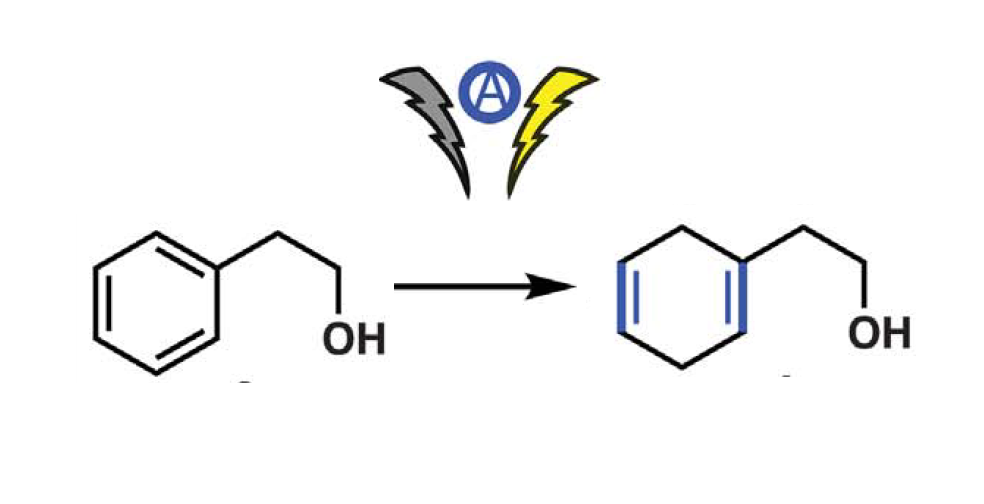
“Perfection does not exist”, someone could say… But Phil S. Baran and his co-workers have made a reality such chemical dreams by applying Li-ion battery chemistry in synthetic organic electroreduction.
Indeed, they demonstrate that it is possible to obtain the same results as from the conventional Birch reduction using a sacrificial anode material in combination with dimethylurea and tris(pyrrolidino)phosphoramide.
What makes this discovery so special?
The basic Birch reduction presents the following characteristics:
- It requires the use of lithium metal (pyrophoric) and ammonia (hazardous),
- It involves cryogenic conditions and
- It liberates hydrogen (explosive).
So it is easy to guess that its scalability is neither sustainable nor safe.
On the contrary, the described new process achieves “strongly reducing conditions (…) in a simple and safe way, at ambient temperature without rigorous exclusion of air and moisture”, as the research team explained in their publication.¹ Moreover, it shows modular scalability, and a wide applicability in terms of compound diversity (from simple arenes to more complex natural products), achieving great chemoselectivity levels.
In this case, the key was found studying Li-ion battery technology, which has taken decades of research and is present daily in our smartphones and laptops. Thus, my reflection is: “Never forget other fields of work, thinking they are too complicated or too simple… Sometimes, the answer is just beside you; nearer than we think. As a matter of fact, research is what makes possible scientific progress; however, it is important to point out that this progress will only be possible if people share their knowledge and work together.
¹ Scalable and safe synthetic organic electroreduction inspired by Li-ion battery chemistry, Byron K. Peters and Co., Science 22 Feb 2019: Vol. 363, Issue 6429, pp. 838–845.