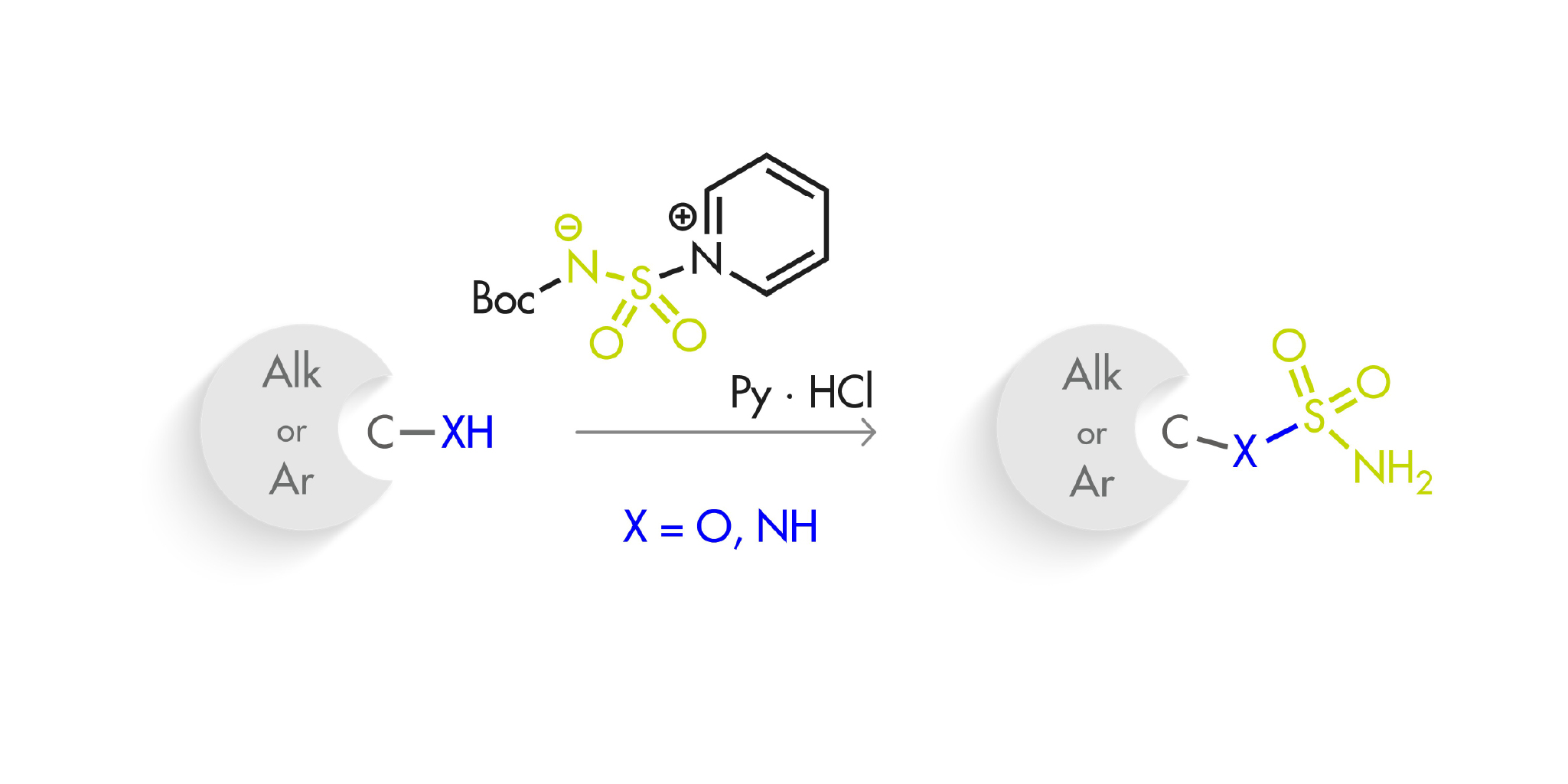
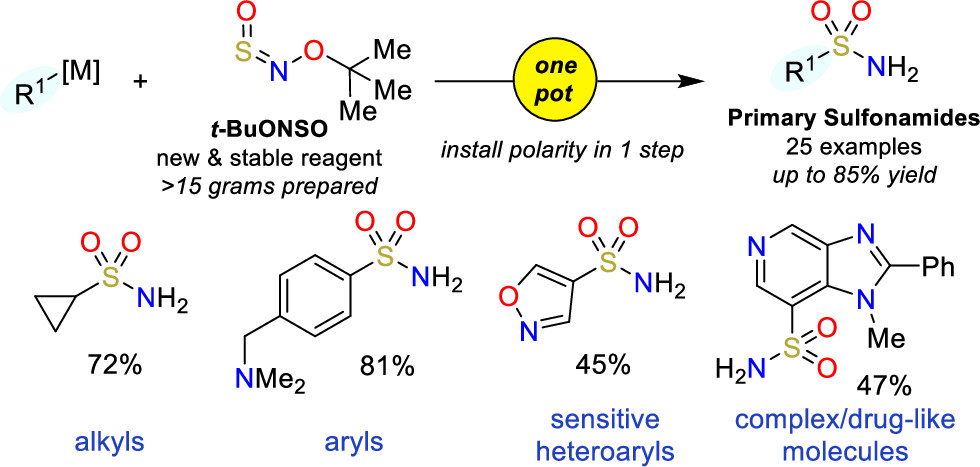
A couple of months back, we featured here a new reagent for the introduction of sulfonamides (One-pot access to primary sulfonamides). Today, we bring you another addition to this field, this time aiming at sulfamides and sulfamates. That is, in this case the reagent is used for the formation of O–S and N–S bonds, instead of a C–S bond.
The protocol published by Wang and Hu (Shanghai Jiao Tong University and Shenyang Pharmaceutical University, China) relies in the use of a Burgess type reagent. For those who do not remember, the Burgess reagent corresponds to 1-ethoxy-N-triethylammoniosulfonyl-methanimidate, which is used as a mild dehydrating agent.
This new sulfamoylation reagent, N-(tert-butoxycarbonyl)-aminosulfonylpyridinium, can be prepared in gram scale using commercially available tert-butyl alcohol and chlorosulfonyl isocyanate, yielding the product as a crystalline, stable solid. When applied to alcohols and phenols, the results range from good to excellent, even with weakly reacting or bulky alcohols. Of course, the products must be subsequently treated with acid (6 M aqueous HCl is enough) to remove the Boc protecting group. The results with primary and secondary amines, both aliphatic and aromatic, are also rather good.
A number of substrates bearing different nucleophilic groups were tested, and no surprises here: nitrogen wins hands down over oxygen, aliphatic alcohols over phenols, and primary alcohols over secondary alcohols. One of the most interesting experiments is the sulfamoylation of a diol derived from a carbocycle for the preparation of Pevonedistat. As expected, the result is a complex mixture, but apparently way better than what is obtained with other reagents.
Preparation of Sulfamates and Sulfamides Using a Selective Sulfamoylation Agent. Org. Lett. 2021, 23(7), 2595–2599. See: 10.1021/acs.orglett.1c00504