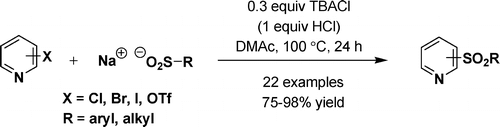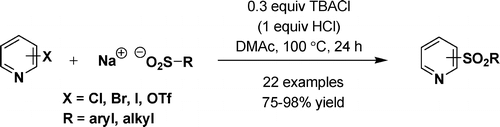A Practical, One-Pot Synthesis of Sulfonylated Pyridines
Sulfonylation of pyridine rings.
Some time ago I had a very interesting talk with a medicinal chemist who told me that ‘today we can prepare everything’. Well, I cannot concur with that statement. Even now, at the beginning of the 21st century, the synthesis of some molecules is complicated and sometimes there are no methods at all. Thanks God, because if everything was so easy fine chemists would not be necessary… Sulfonylated pyridines are among those hard to prepare molecules due to several reasons (pyridine ring is electron deficient, typical oxidations of sulfides are sometimes troublesome, and so on).
The chemists from Merck USA have tackled this problem and solved it with their usual skills. The protocol published by Maloney et al. is short and bomb proof, as expected of the work done by process chemists. After conducting a thorough catalysis screen exploring Pd and Cu sources, ligands, additives and solvents they found out that the combination of tetrabutylammonium chloride (TBACl) as an additive and dimethylacetamide (DMAc) yields the expected product. The use of the PTC enhances the reaction rate, since without it the sulfinate used as nucleophile is less soluble and the yield drops by a five fold. The reaction works with almost any chloropyridine (though better, as expected, with electron-deficient pyridines) in yields between 75% and 98%. Other halides can work if the pyridine is reactive enough.
Org. Lett., 2011, 13 (1), pp 102–105. See: 10.1021/ol102629c