The 2022 Chemistry Nobel Prize has been awarded to Carolyn R. Bertozzi, Morten Meldal, and K. Barry Sharpless “for the development of click chemistry and bioorthogonal chemistry”.
Looking back now, the year 2001 was particularly remarkable for Barry Sharpless. He not only received a share of the Chemistry Nobel Prize for his work on asymmetric catalysis, but he also co-authored the seminal paper on the work that has just earned him a second Nobel Prize in the same category.
The article published in Angewandte Chemie International Edition had an oddly simple but very illustrative title: Click Chemistry: Diverse Chemical Function from a Few Good Reactions. The central thesis of the article is that nature manages to create all its diversity with a handful of very simple but extremely robust reactions that fall into just 3 categories: Nucleophilic Opening of Spring-Loaded Rings, Cycloaddition Reactions, and what they called “Protecting Group” Reactions. In all of them, two blocks react in a wonderfully selective and efficient way, rendering the comparison to the clicking of Lego® blocks almost inevitable.
The paper defined a series of criteria a transformation must meet in order to be considered click:
“The reaction must be modular, wide in scope, give very high yields, generate only inoffensive byproducts that can be removed by nonchromatographic methods, and be stereospecific (but not necessarily enantioselective). The required process characteristics include simple reaction conditions (ideally, the process should be insensitive to oxygen and water), readily available starting materials and reagents, the use of no solvent or a solvent that is benign (such as water) or easily removed, and simple product isolation. Purification—if required—must be by nonchromatographic methods, such as crystallization or distillation, and the product must be stable under physiological conditions.”
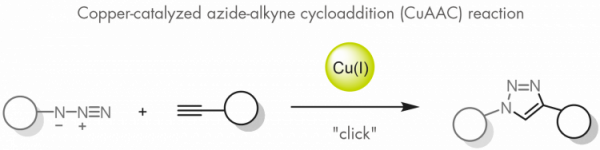
Shortly afterwards, Morten Meldal and Barry Sharpless – albeit independently – presented what is probably the most widely known click reaction: the copper catalyzed azide-alkyne cycloaddition, which is used today in the development of pharmaceuticals, for mapping DNA, and in the preparation of fit-for-purpose materials.
Carolyn Bertozzi, this year’s third laureate, took click chemistry into a nearly uncharted realm, using those reactions to study cells inside and out. This feat included the development of bioorthogonal reactions that do not disrupt cellular chemistry. These reactions can now be used to improve the targeting of cancer pharmaceuticals, as currently being tested in clinical studies.
What a great example of how the discovery and development of new reactions can radically change other sciences!
Jacobo Cruces
CSO
Links of Interest
- Nobel Prize in Chemistry 2022
- Click Chemistry: Diverse Chemical Function from a Few Good Reactions, Angew. Chem. Int. Ed. 2001, 40(11), 2004-2021.
- Our tribute to Chemistry Nobel Prize winners