Looking for new customers in France, Belgium, United Kingdom, and other European countries, I realized that the number of pharmaceutical companies working on Small Chemical Entities (SME)is reducing, with the market moving towards biologics . So I wondered whether the future will be chemical or biological.
Biologics existed for a long time: for example, preventive vaccines made up of inactivated or attenuated forms of the pathogens (virus or bacteria). Blood products and hormones such as insulin have also been represented in the market for many years. Nowadays we can add large peptides and recombinant proteins, as well as what are known as therapeutic vaccines.
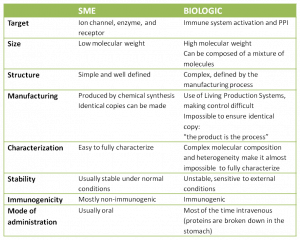
Biologics initially emerged to answer a specific need: how to break a protein-protein interaction (PPI) characterized by large and often flat surfaces with few charged pockets, making it difficult for chemical entities to bind. But what is the difference between biologics and SME? Is one class significantly better than the other?
It seems that the “only” strong point of biologics is that they can interact with challenging targets or activate specific mechanisms of action which have thus far eluded small molecule drugs. But it also looks as if biologics are delivering a better overall economic return than SME for several reasons:
- The treatment is expensive, which means that the financial return per patient is very high.
- Studies also show that the rate of attrition for biologics is lower than for small molecules.
- Moreover, once the patents expire, these developers face less competition from biosimilars than small molecule producers do from chemical generics.
Nevertheless, manufacture is still a very delicate point. I recently read that the ideal product would be a chemical molecule big enough to break PPI without the constraints of a biologics . And, hardly surprisingly, several companies are already moving in that direction. We are working with HQL with the aim of developing new PPI and we have also been approached to work on several PPI disruptions.
Nowadays, the types of biological therapy have expanded to include nanoparticles, fusion proteins, immune-conjugates, etc…. These latest technologies use a combination of biologicals and chemical entities. These new pharmaceutical products involve chemistry in two ways. Firstly, the proper conjugate is a chemical product and, in that case, the “biological” is used as carrier to help target the right cells. Secondly, the two combined products are linked using chemical linkers. These specific linkers will require a certain level of development as they may, for example, help with the release of the active product.
The design and synthesis of these new chemical products will be a new challenge for the chemical company chemists and this novel paradigm will undoubtedly raise new issues on which our regulatory agencies will have to work hard. But chemistry is undoubtedly still well positioned in the race for new pharmaceutical developments.