New Palladium-Catalyzed Methods for Ester Formation
For the fans of esters, we have here a couple of palladium methods for the introduction of a carboxy moiety.
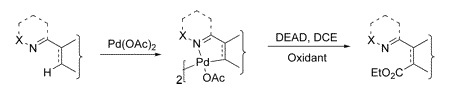
Common methods for the introduction of a carboxy moiety into a non-functionalized substrate (i.e., functionalization of a C–H) involve usually metallation and trapping with a suitable electrophile. For sure, other methods of C–H functionalization would be very welcomed. We present here one recent paper addressing that need. The work by Yu et al. (Hong Kong, China) opens a new way into esters using DEAD. The method has application only over 2-arylpyridines and structurally-related substrates, and involves the use of Pd(OAc)2 to form a palladacycle, which is in turn treated with DEAD and an oxidant in DCE at 100 °C. Oxone works fine as the oxidant, but Cu(OAc)2 or K2S2O8 can also be used. Yields are typically over 70%. Leaving aside the structural requirements, the reaction is compatible with many functional groups.
J. Am. Chem. Soc., 2008, 130 (11), pp. 3304–3306. See: 10.1021/ja710555g
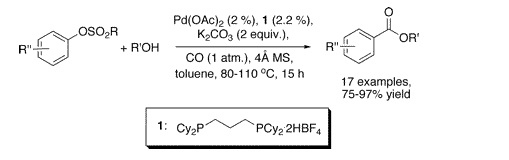
Our second example is a work by Buchwald et al. (Cambridge, Mass, USA), a follow-up of their efforts in the carbonylation of cheap aromatic substrates. This time, they have selected aryl tosylates and mesylates, which are possible substrates for metal-mediated couplings but are often neglected in favor of the triflates. The conditions developed involve the use of CO at room pressure, n-BuOH, Pd(OAc)2, a ligand, and K2CO3 in toluene. Two interesting points about these conditions are that no pressure is needed, allowing thus the use of a balloon filled with CO, and that the ligand is commercially available as a salt. Yields are excellent and a wide range of substrates, including some heterocycles, are presented.
J. Am. Chem. Soc., 2008, 130 (9), pp. 2754–2755. See: 10.1021/ja711449e