A Practical Lewis Base Catalyzed Electrophilic Chlorination of Arenes and Heterocycles
Mild chlorination method of arenes and heteroarenes.
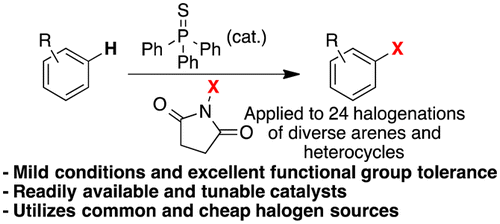
Here you have one of those small advances in organic synthesis that sometime come handy when you are preparing a chemical series and you want to try the chlorine in the system. For those of you that have not done any chlorination (not the preparation of a benzylic chloride, thanks), it is a tricky reaction. For starters, you can’t do it using chlorine. The most common reagent, N-Chlorosuccinimide (NCS) does not work very well, and most times the system simply does not react. And then you have the always present selectivity problem. Very recently the group of Baran presented a new chlorination reagent, chloro-bis(methoxycarbonyl)guanidine (CBMG) that works much better and it is the best current alternative.
This work by Gustafson (San Diego State University, CA, USA) is another step towards a fast, mild, friendly chlorination. In summary, they have tried a number of catalysts and finally selected the commercially available triphenylphosphine sulfide as a good alternative (not the best, as they note there is still room for improvement). They carried out the chlorination using mainly azaindoles and indoles as substrates (8 examples), with excellent results. The worst isolated yields is a 55%, in a substrate where they are introducing four chlorine atoms. In several azaindoles, the isolated yields are between 66 and 81%. Moving to normal arenes gives also good results, and they demonstrate that phenols, boronic acids, isotiocyanates and interestingly, a Boc protected amine, can be sucessfully chlorinated. Tough NCS is the usual reagent, they compare also with CBMG. Finally, they apply the same catalysis to the bromination and iodination (NBS and NIS) of some selected substrates. Some reactions have also been done in gram scale. Not bad at all.
Org. Lett. 2015, 17 (4), pp 1042-1045.
See: 10.1021/acs.orglett.5b00186
Freak Corner. Chlorine was first used as a chemical weapon in 1915, during the Second Battle of Ypres. After the first and succesful result, both sides used it, but changed to better things like phosgene and mustard gas. Its use as a weapon was pioneered by the German chemist Fritz Haber, who was awarded the 1918 Nobel Prize in Chemistry for his work in the Haber-Bosch process for the production of ammonia. Charming.