Direct Catalytic N?Alkylation of Amines with Carboxylic Acids
Ask a medicinal chemist how many times he/she used the reductive amination. The answer will probably be ‘many times’ or something similar. The reductive amination is one of the best and therefore most used ways to prepare substituted amines. As any other reaction it has limits, since the use of a reducing agent simply precludes the presence of some moieties. The classical paper on this subject was published by Abdel-Magid et al. more than 20 years ago (see 10.1021/jo960057x), and they published a more focused review on the use of a specific reducing agent in 2006 (see 10.1021/op0601013).
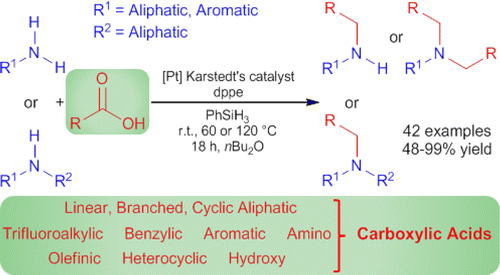
Now, Mathias Beller and coworkers (University of Rostock, Germany) have published a new way to carry out a reductive amination, using amines… and carboxylic acids. The protocol uses PhSiH3 as reducing agent, and the trick is done by Karstedt’s catalyst, a Platinum-based catalyst with the aid of the phosphine dppe. Both the catalyst and the phosphine are commercially available.
The protocol works with aliphatic and aromatic amines (maybe too many anilines), and many types of carboxylic acids, including pivalic acid. Some protecting groups like Cbz, Boc, conjugated acids, etc., stand the protocol.
The method has obvious limitations (i.e., you can access only amines with no branching in the alpha position), and there are still many things to be tried (heterocycles, hindered amines…). But all considered, a very attractive way to prepare amines.
J. Am. Chem. Soc. 2014, 136(-), pp 14314–14319.
See: 10.1021/ja5093612