Anhydrous Hydration of Nitriles to Amides Using Aldoximes as the Water Source
Selective method for the anhydrous hydrolysis of nitriles.
Oxymoron: figure of speech that combines normally contradictory terms. Attending to this definition, anhydrous hydrolysis can be considered without a doubt an oxymoron. Unless, of course, you are using another chemical to transfer the same atoms you would transfer with water.
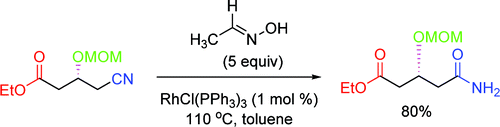
This is the base of a paper by Chang and Lee (KAIST, Korea). In the search of a method for the rearrangement of aldoximes to amides, they have found that a rhodium catalyst promotes the desired transformation, which is accelerated by the addition of a nitrile. A conceptual reasoning led them to apply similar conditions for the mild and neutral hydrolysis of nitriles with great results. In a typical reaction, a nitrile is reacted with acetaldoxime and RhCl(PPh3)3 (1 mol%) in toluene at 110 °C. The yields obtained are excellent and the scope of the reaction is quite broad. Most important, the method is selective and leaves unaltered other ester moieties in the substrate. Both the oxime and the catalyst are commercial (yes, it is the Wilkinson’s catalyst), and the byproduct of the hydrolysis, acetamide, is water soluble.
Org. Lett., 2009, 11 (24), pp 5598–5601. See: 10.1021/ol902309z