An Expeditious and Environmentally Benign Methodology for the Synthesis of Substituted Indoles from Cyclic Enol Ethers and Enol Lactones
An interesting application of an old reaction in modern times.
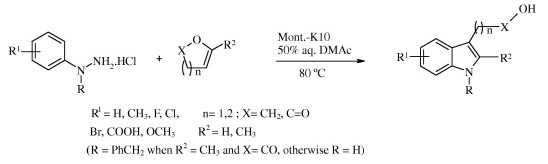
The Fischer indole synthesis has maintained its prominent role among the various approaches to these compounds. A wide range of hydrazine and ketone starting materials are available and the procedure is easy, atom-economical, versatile, and can be modulated in several ways. Many modifications of the reaction have been described, usually involving a change in the acid catalyst. Very recently, it has been shown that enol ethers and enol lactones can serve as ketone equivalents.
In the search for a more environmentally friendly reaction, the group of Singh (Mumbai, India) reports greener conditions. From a screening of several solid acids, Montmorillonite K10 emerged as one of the best catalysts. The final protocol involves a hydrazine and an enol ether with Mont. K10 in water (or a mixture of water/DMAC). Twelve examples are described with excellent yields.
Tetrahedron Lett., 2008, 49(20), pp 3335–3340. See: 10.1016/j.tetlet.2008.02.107