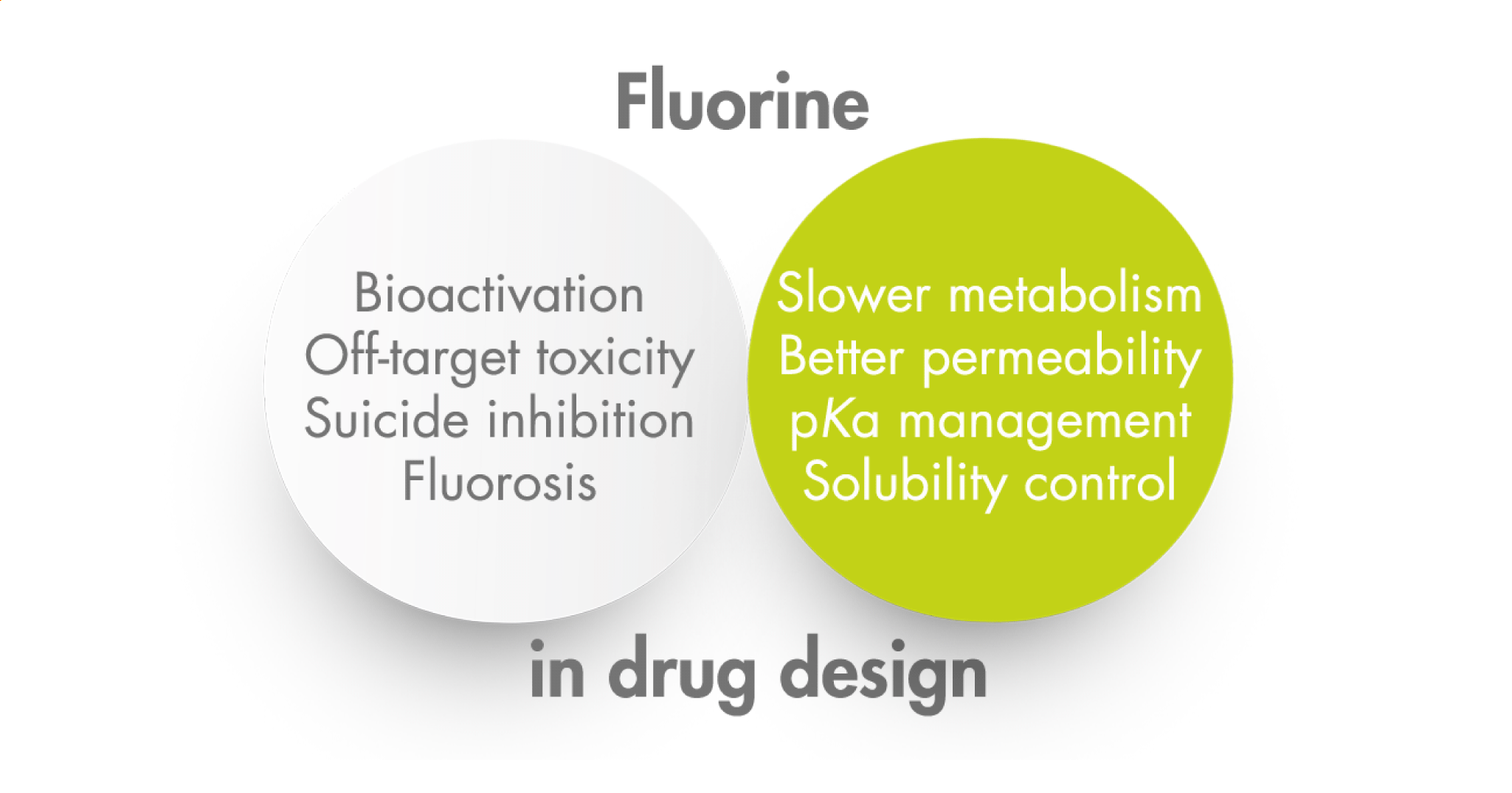
The first drug incorporating fluorine in its structure was commercialized in 1955. Since then, the presence of fluorine in drugs and agrochemicals has become rather common. Indeed, numerous articles have been published on the importance of fluorine in medicinal chemistry and the many advantages it confers in terms of potency, selectivity, metabolic stability, and solubility, among others. There is also extensive literature covering the latest synthetic fluorine incorporation methods, both for the introduction of a single atom and for more complex groups: trifluoromethyl, difluoromethyl, trifluoromethoxy, and many more.
However, despite the strength of the C–F bond, certain compounds produce toxic effects through metabolic mechanisms that are not always obvious. This Perspective by Johnson et al. (Bristol Myers Squibb Company) places special emphasis on the metabolism of fluorinated compounds, examining the degradation and toxicology of various classes of products.
Metabolic and Pharmaceutical Aspects of Fluorinated Compounds, J. Med. Chem. 2020, 63(12), pp. 6315–6386. See: 10.1021/acs.jmedchem.9b01877