Overcoming the limitations of directed C–H functionalizations of heterocycles
C-H activation on heterocycles, avoiding the activation of the C-H adjacent to the heteroatom.
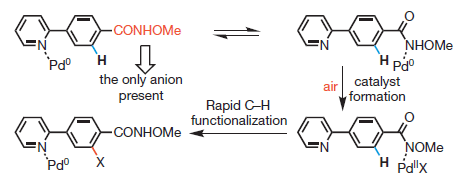
Let’s say it again, just in case: C-H activation is a hot topic in organic synthesis. In some way it represents the culmination of several decades of organocatalysis. We have learnt how to use many different Xs as coupling partners: Iodides, Bromides, Triflates, Chlorides, Phosphonates, Carboxylates… We can use even fluorides (see a previous entry of the blog). The logical conclusion is to overcome the need of the X, activating directly the C-H bond. But there are still some limitations, which some groups are trying to overcome. Dai et al. (Scripps, USA & Sanghai, China) make use of a Weinreb amide and the combination of a Palladium catalyst with t-Butylisonitrile to force the activation of C-H bonds not adjacent to the heteroatom already present in the heterocycle. The work deserves some attention, though we are still a long way from a general protocol.
Nature 2014, 515, pp 389-393.
See: 10.1038/nature13885