A General and Efficient Method for the Synthesis of Benzo-(iso)quinoline Derivatives
A new method for the preparation of benzoquinolines and benzoisoquinolines.
We have said it before, but we will repeat it now: any new method for the synthesis of heterocycles is worthwhile. Bonus points are given if the method is one-pot, flexible, and uses commercially available raw materials.
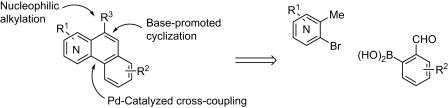
The method published by Mamane et al. (Nancy, France) scores high. The synthesis of fused benzoquinolines and benzoisoquinolines is usually tedious and troublesome. This new approach involves a first coupling of an ortho-methylhalopyridine (which provides the nitrogen of the ring system) with an aldehyde through a Suzuki or Negishi coupling. The biaryl system is then cyclized using a base (t-BuOK) to give the fused heterocycle. The new ring can be functionalized if an intermediate step of base/electrophile trapping is performed, provided the aldehyde is protected.
Examples with seven different ortho-methylhalopyridines are included. The expected compounds are obtained in yields from good to excellent. This method offers a great potential, being only restricted by the availability of ortho-methylhalopyridines and boronic benzaldehydes or benzaldehydes suitable to be transformed into Negishi partners.
Tetrahedron, 2008, 64, pp. 10699–10705. See: 10.1016/j.tet.2008.09.015