Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Alkyl Ethers: Enantioselective Synthesis of Diarylethanes
Ni-catalyzed replacement of methoxy groups.
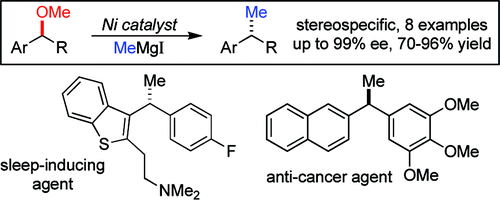
I must admit that when I first saw the paper graphical abstract I thought it was a mistake. ‘Hey, they have missed the oxygen somewhere’. That lasted the few seconds needed to read the abstract and see that in fact the reaction is designed to do exactly that: to change a methoxy group into a methyl group, so ‘missing’ the oxygen.
Obviously the reaction is a bit more complex than that. It is not a question of missing, but in fact cross-coupling a suitable partner with the methyl source in the presence of the right catalyst. The work by Jarvo et al. (Irvine, CA, USA) has determined that in fact this can be done using a Nickel catalyst with a widespread, cheap ligand, BINAP and the methyl source is methylmagnesium iodide.
In a typical experiment, Ni(cod)2 (5 mol%) and racemic BINAP (10 mol%) are introduced in a vial with toluene. The substrate and the methylmagnesium iodide are then added sequentially and the mixture stirred at r.t. for 24 h. Though the conditions involve the use of a glove box, probably the procedure can be adapted to be used with Schlenk techniques. The results obtained are excellent both in yield and ee in most cases, as the reaction proceeds with inversion of configuration and high stereochemical fidelity. When BINAP shows not to be the best ligand, DPEphos and Xantphos, both commercially available, can be used as alternatives.
J. Am. Chem. Soc., 2011, 133 (3), pp 389–391. See: 10.1021/ja108547u