Versatile Pd(II)-Catalyzed C–H Activation/Aryl–Aryl Coupling of Benzoic and Phenyl Acetic Acids
It is always interesting to see advances being made in a field I am very fond of.
C–H activation is a thriving field in synthetic chemistry. In previous issues of our newsletter (see here this main article), we have commented on the advantages of this approach. More and more papers are being published reporting C–H activation in different substrates. I can only say that it is a pleasure to see that some advances are being made in a chemistry topic I am very fond of, since I spent most of my Ph.D. working with similar compounds.
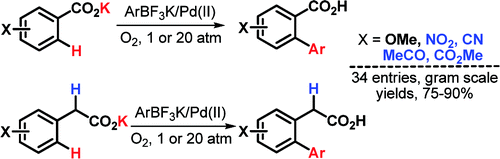
This paper from Yu et al. (Scripps, CA, USA) expands his previous work on the ortho-coupling of benzoic acids. The new conditions are not only easier and more robust, but can also be applied to rich and poor benzoic acids and, for first time, to phenylacetic acids. This is important because these acids are synthetically very useful, but the presence of acidic a-hydrogens is not tolerated in many reactions.
To overcome the problems previously discovered, the method relies on the use of much more reactive boron derivatives, trifluoroborates. Addition of the potassium aryltrifluoroborate, Pd(OAc)2, benzoquinone, and K2HPO4 to the benzoic acid in tert-butanol at 100 °C in a vessel pressurized with O2 or air to 20–30 atm for 24 h yields the desired coupled products in good yields. If the reaction is carried out instead at ambient pressure the reaction time increases to 48–72 h, but it works the same.
Phenylacetic acids are rather troublesome (as usual, haha) and pressurization is needed to complete the reaction unless you can wait 6 or 7 days. One additional problem with phenylacetic acids is biarylation, but the presence of an a-substituent provides sufficient steric hindrance to induce monoselectivity.
The products can be further transformed into esters or cyclic ketones by conventional chemistry. Many examples are reported, including some couplings with 3-pyridine trifluoroborates. The main shortcoming of this new protocol is the use of pressurization, but the authors are improving the efficiency of this reaction under 1 atm of air.
J. Am. Chem. Soc., 2008, 130 (52), pp 17676–17677. See: 10.1021/ja806681z