Cross-coupling of aryl esters catalyzed by Ni
Pivalates are usually considered protecting groups. Change your mind.
Transition metal-catalyzed cross-coupling reactions being a powerful method for constructing carbon–carbon and carbon–heteroatom bonds is not surprising at all. Cross-coupling protocols for almost any conceivable group have been developed, but medicinal chemists are quite focused in a small number of reactions with a handful of reacting groups. When a reaction is needed, we automatically start thinking “how can we go from this group to this other?”. However, recently, some efforts are being done to develop conditions that render such automatic thinking useless. The trick is to use groups that are not usually considered as reacting, but protecting.
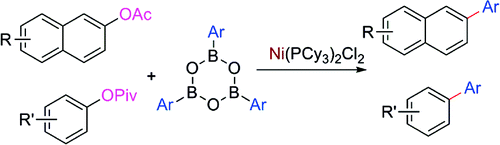
Two papers have been recently published on this topic. The first one is a work by Shi et al. (BNLMS, Beijing, China), aimed at developing greener and cheaper conditions for the Suzuki reaction. That is, the use of catalysts different from Pd, use of more available aryl partners and less toxic and even recoverable subproducts. After some research, they found that the use of a Ni(II) precatalyst can be used to carry out the coupling of a boronic acid with an acyl protected hydroxynaphtalene. The boron source used in the reaction are boroxines. The most efficient acyl group is the acetyl, being a compromise between low steric hindrance and stability to hydrolysis under the reaction conditions. A wide pool of boroxines was also tried, demonstrating that the reaction is compatible with a suitable range of boroxines. The protocol uses NiCl2(PCy3)2 with K3PO4 as the base in dioxane/water at 110 °C. Importantly, the authors found that the amount of water played a crucial role in promoting the transformation. The conditions were subsequently applied to pivalates derived from phenols with similar results. No examples with heterocycles, excepting a chromen-4-one, are reported.
The second paper is a work by Garg et al. (UCLA, USA), submitted only a few weeks latter. In a very similar way, they have found that a Ni(II) precatalyst can be used to carry out the coupling of a boronic acid with an acyl protected phenol. The most striking aspect of the method is that the acyl group is a pivalate group, which is usually regarded as a hindered protecting group. This can be specially important when other C–O activating groups (i.e., triflates) cannot be prepared or are unstable. The protocol presented is quite similar to the one reported by Shi in the previous paper, also using NiCl2(PCy3)2 with K3PO4 as the base in toluene at 80–110 °C. Under these conditions, a range of naphtyl and phenyl pivalates are coupled to phenylboronic acid in good to excellent yield (58–92%). One example of a heterocycle (a carbazole) is also provided. Although some more examples would be welcomed, it seems the reaction is robust enough to work with other phenylboronic acids.
As a bonus, the protocol can be done in a one-pot manner by preparing the pivalate with K3PO4 as the base in toluene, and adding to the reaction mixture the boronic acid and Ni catalyst. This catalyst does not react with other halides, so orthogonal cross-coupling reactions can be done, something specially interesting for library synthesis.
An important note: The authors report that the Ni(II) precatalyst will soon be commercially available from Strem Chemicals Inc. (catalog #28-0091) but, if in the meantime, you want to try the reaction, it can be prepared in multigram quantities following a simple one-step protocol.
Biaryl Construction via Ni-Catalyzed C-O Activation of Phenolic Carboxylates
J. Am. Chem. Soc., 2008, 130 (44), 14468–14470. See: 10.1021/ja8056503
Cross-Coupling Reactions of Aryl Pivalates with Boronic Acids
J. Am. Chem. Soc., 2008, 130 (44), pp 14422–14423. See: 10.1021/ja806244b