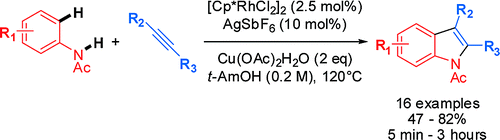Indole Synthesis via Rhodium-Catalyzed Oxidative Coupling of Acetanilides and Internal Alkynes
A new preparation of 2,3-disubstituted indoles from o-haloanilines and internal alkynes.
Indoles are probably one of the most studied heterocycles, with plenty of synthetic methods available. However, the ubiquity of this scaffold and its importance makes new additions worthwhile. We have published before in our newsletter new methods for the preparation of substituted indoles, but usually monosubstituted in the C2 or C3 positions.
The method published by Fagnou’s group (Ottawa, Canada) is directed to the regioselective preparation of indoles disubstituted in the C2 and C3 positions. Thus, a mixture of an ortho-substituted N-acethylhaloaniline is reacted with an alkyne in the presence of [Cp*RhCl2]2 (2.5 mol%), AgSbF6 (10 mol%) and 2.1 equiv of Cu(OAc)2·H2O in t-AmOH at 120 °C for 1 h. The regioselectivity of the reaction is quite good, with the most bulky substituent going into the C2 position. Some substituents like esters, chlorine and others are tolerated. The acetyl used as protecting group can be smoothly hydrolyzed using KOH or K2CO3 in MeOH/DCM at room temperature.
J. Am. Chem. Soc., 2008, 130 (49), pp 16474–16475. See: 10.1021/ja806955s